
Link to Article:https://doi.org/10.1089/respcare.12363
Short Summary:
This study demonstrates that SafeBVM’s Sotair flow-limiting device significantly improves manual ventilation control, ensuring peak airway pressures remain within safe limits. Conducted with 41 respiratory therapy students, the study compared manual ventilation using the Sotair device to mechanical ventilation across normal and decreased lung compliance settings.
Key findings:
- Comparable peak inspiratory pressures between manual ventilation with Sotair and mechanical ventilation (15 vs 13 cmH2O with normal lung mechanics; 23 vs 23 cmH2O with decreased lung compliance).
- Effective tidal volume (VT) delivery, with ventilation performance slightly lower with Sotair (452 vs 474 for normal lung compliance; 312 vs 460 ml for low compliance) compared to mechanical ventilator.
- Participants effectively adapted to changing lung compliance, demonstrating the device’s ability to enhance manual ventilation consistency.
- The flow-limiting feature prevented excessive inspiratory pressures, potentially reducing the risk of barotrauma and gastric insufflation.
Conclusion:
The Sotair device bridges the gap between manual and mechanical ventilation, providing controlled, safe, and effective manual ventilation. This study reinforces its potential to improve patient outcomes in both emergency and controlled settings, making it a valuable innovation for respiratory care.
Reference:
Kumar P, Culbreth R, Gardenhire DS, Slutsky AS, Wu YJ, Kendall MC, Brady MF. Manual ventilation performance with safety device in normal versus decreased lung compliance: a single-center simulation study. Respir Care. 2025;00(00):1-4. doi:10.1089/respcare.12363.
Link to Article:
https://innovations.bmj.com/content/7/2/297
Short Summary:
This study highlights the critical role of SafeBVM’s Sotair device in enhancing manual ventilation when mechanical ventilators are unavailable. Conducted during a period when there were concerns of ventilator shortages, the study assessed the effectiveness of manual ventilation with and without the Sotair device over extended periods.
Key Findings:
- Reduced Risk of Barotrauma: The Sotair device significantly lowered peak inspiratory pressures (14.9 cm H₂O vs. 17.2 cm H₂O without Sotair, p<0.0001), reducing the risk of ventilator-induced lung injury.
- Improved Tidal Volume Control: Manual ventilation with the Sotair device resulted in more consistent tidal volumes (mean (+SD) Vt of 536+81 with Sotair vs 565+129 ml without Sotair), mitigating risks of over- and under-ventilation.
- Feasibility for Extended Use: The study confirmed that manual ventilation with Sotair is a viable alternative in critical situations, providing a safer and more controlled method for delivering ventilation compared to traditional manual resuscitation.
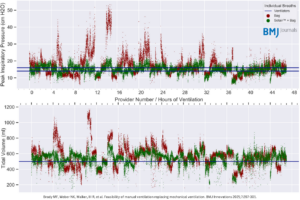
Conclusion:
The Sotair device bridges the gap between manual and mechanical ventilation, optimizing safety and performance. By limiting excessive pressures and ensuring consistent tidal volumes, the device enhances the quality of manual ventilation. This research underscores Sotair’s potential to improve patient care while reducing risks associated with traditional bag-valve-mask ventilation.
Reference:
Brady MF, Weber NK, Walker III R, Holley JE, Ni SA, Young S, Monhollon ED, Carpenter RS, Tsao JW. Feasibility of manual ventilation replacing mechanical ventilation. BMJ Innov. 2021;0:1-5. doi:10.1136/bmjinnov-2020-000524.
Link to Article:
https://doi.org/10.1080/10903127.2024.2425372
Short Summary:
This study compared the SafeBVM Sotair flow-regulating safety device against traditional pop-off valves (set at 25 cmH₂O and 40 cmH₂O) when forceful breaths were delivered to mechanical test lungs simulating healthy and disease-state conditions.
Key Findings:
- The Sotair device consistently maintained lower and safer peak inspiratory pressures (PIP) than the pop-off valves across all conditions.
- Healthy lungs: 10.6 cmH₂O (Sotair) vs. 33.1 cmH₂O (25 cmH₂O valve) and 41.6 cmH₂O (40 cmH₂O valve).
- Obstructive disease: 35.5 cmH₂O (Sotair) vs. 44.4 cmH₂O (25 cmH₂O valve) and 56.5 cmH₂O (40 cmH₂O valve).
- Restrictive disease: 22.2 cmH₂O (Sotair) vs. 37.6 cmH₂O (25 cmH₂O valve) and 47.3 cmH₂O (40 cmH₂O valve).
- Sotair provided controlled and effective tidal volumes (TV) while preventing excessive pressures, ensuring adequate ventilation in all lung conditions.
- Pop-off valves often failed to release at designed pressures, reducing their reliability in prehospital settings.
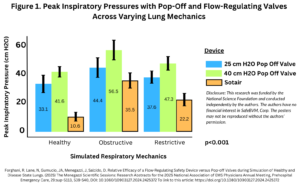

Conclusion:
Pop-off valves inconsistently released at the set pressures, often resulting in higher-than-anticipated inspiratory pressures. In contrast, Sotair delivered consistent tidal volumes across healthy, restrictive, and obstructive conditions. Additionally, the flow-regulating device maintained tidal volumes at lower peak inspiratory pressures in all conditions.
Reference:
Forghani, R, Lane, N, Gumucio, JA, Menegazzi, J, Salcido, D. Relative Efficacy of a Flow-Regulating Safety Device versus Pop-off Valves during Simulation of Healthy and Disease-State Lungs. (2025) The Menegazzi Scientific Sessions: Research Abstracts for the 2025 National Association of EMS Physicians Annual Meeting, Prehospital Emergency Care, 29:sup-S113, S39-S40, DOI: 10.1080/10903127.2024.2425372
Link to Article:
https://doi.org/10.1080/10903127.2024.2425372
Short Summary:
This study compared manual ventilation using the SafeBVM Sotair flow control valve (FCV) against a mechanical transport ventilator under simulated lung conditions, including healthy lungs, obstructive disease, and restrictive disease.
Key Findings:
- Manual ventilation with Sotair provided PIP and tidal volumes (Vt) comparable to a mechanical ventilator, proving its effectiveness as a safer alternative when ventilators are unavailable.
- Healthy lungs: PIP 10.6 cmH₂O (Sotair) vs. 13.6 cmH₂O (ventilator); Vt 654 mL (Sotair) vs. 1,037 mL (ventilator).
- Obstructive disease: PIP 35.5 cmH₂O (Sotair) vs. 17 cmH₂O (ventilator); Vt 710 mL (Sotair) vs. 747 mL (ventilator).
- Restrictive disease: PIP 22.2 cmH₂O (Sotair) vs. 19.6 cmH₂O (ventilator); Vt 711 mL (Sotair) vs. 801 mL (ventilator).
- The Sotair device prevented excessive inspiratory pressures
Conclusion:
The study confirmed that SafeBVM’s Sotair device provided ventilation performance that was similar to that of a mechanical ventilator.
Reference:
Salcido, DD, Forghani, R, Lane, N, Gumucio, JA, Menegazzi, JJ. A Comparison between Manual Ventilation with a Flow Control Valve versus a Mechanical Transport Ventilator. Prehospital Emergency Care, S67-S68. (2025) The Menegazzi Scientific Sessions: Research Abstracts for the 2025 National Association of EMS Physicians Annual Meeting, Prehospital Emergency Care, 29:sup1, S1-S113, S67-S68DOI: 10.1080/10903127.2024.2425372
Short Summary:
This study involved 217 first responders from fire departments in Tennessee. Participants performed 60 seconds of manual ventilation on a simulated lung without the Sotair device. After a brief educational intervention on using the Sotair flow-rate limiting device, they repeated the 60-second ventilation session with Sotair attached to the manual resuscitator bag. The study compared peak inspiratory pressures, tidal volumes, and respiratory rates before and after the intervention.
Key Findings:
- Lower Peak Inspiratory Pressures: Sotair reduced peak pressures (15.7 cmH₂O vs. 17.6 cmH₂O, p < 0.01).
- More Controlled Tidal Volumes: Sotair provided lower tidal volumes (525 mL vs. 594 mL, p < 0.01).
- Improved Ventilation Consistency: After a brief educational intervention, providers ventilated more consistently with Sotair, demonstrating its ease of adoption and effectiveness in real-world emergency settings.

Conclusion:
The Sotair device significantly improves manual ventilation performance, reducing variability and risks associated with excessive pressures and tidal volumes while ensuring more consistent, controlled, and safer ventilation. This study highlights Sotair’s useful role in optimizing first responder ventilation performance.
Reference:
Kumar P, Holley JE, Justice JM, Slutsky AS, Brady MF. Manual ventilation performance in first responders using a flow-rate limiting device (Sotair). Presented at: EMS World Expo’s International Scientific Symposium in partnership with UCLA’s Prehospital Care Research Forum; September 2024; Las Vegas, NV.
Link to Article:
https://doi.org/10.1080/10903127.2023.2273890
Short Summary:
This porcine experimental study demonstrates that SafeBVM’s Sotair flow-limiting device significantly improves manual ventilation safety during prolonged transport, reducing the risks associated with excessive airway pressures and flow rates.
Key Findings:
- Reduced Peak Inspiratory Pressure (PIP): The Sotair device effectively lowered PIP to 31.2 cmH₂O (95% CI: 29.9–32.4) compared to 72.8 cmH₂O in the sham device group (p<0.0001), minimizing the risk of barotrauma and lung injury.
- Controlled Inspiratory Flow Rate (IFR): Sotair maintained a more stable IFR (20.7 L/min, 95% CI: 19.1–22.4), significantly lower than the sham device group (62.4 L/min, 95% CI: 55.8–69.1, p<0.0001), which would help prevent lung overdistension.
- Consistent Performance in BVM & ETI Settings: The Sotair device effectively controlled ventilation parameters in both bag-valve-mask (BVM) and endotracheal intubation (ETI) settings, ensuring safer ventilation delivery across prehospital scenarios.
Conclusion:
The SafeBVM Sotair device improved manual ventilation by preventing dangerously high airway pressures and excessive flow rates, reducing lung injury risk during simulated prolonged transport. These results demonstrate that Sotair enhances ventilation safety and consistency, making it a very useful airway management tool for EMS providers in both prehospital and transport settings.
Reference:
Salcido DD, Gumucio JA, Jeong KW, Stewart H, Shekhani H, Slutsky AS, Prabhudesai P, Ventrapragada A, Lane N, Defilippi D, Menegazzi JJ. Efficacy of a ventilatory safety accessory for use with manual ventilations during simulated prolonged transport: A porcine experimental study. Prehosp Emerg Care. 2024. doi:10.1080/10903127.2023.2273890.
Short Summary:
This study highlights the effectiveness of the Sotair device in improving manual ventilation safety and performance through an educational intervention for EMS providers.
Key Findings:
- Significantly Lower Peak Inspiratory Pressures (PIP):
- Use of the Sotair reduced PIP by 4.1 cmH₂O (19.3 ± 5.80 cmH₂O vs. 15.3 ± 2.44 cmH₂O, p < 0.0001), minimizing the risk of barotrauma and lung injury.
- Reduced Gastric Insufflation Risk: 36.5% of standard BVM breaths exceeded 20 cmH₂O, compared to only 0.25% of breaths with Sotair, demonstrating its ability to prevent excessive pressures that contribute to aspiration risks.
- Improved Ventilation Consistency: Minute ventilation was significantly lower with Sotair (6,312 cc vs. 7,550 cc), indicating better control over ventilation volumes.
- Educational Intervention Enhanced Safety: After a brief training session, 65.6% of providers had positive feedback about the educational material vs 34.4% who were neutral, reinforcing that it is relatively easy to learn how to use the Sotair and use it effectively.

Conclusion:
The Sotair device significantly enhanced manual ventilation safety, reducing peak pressures and gastric insufflation risk while ensuring more controlled and consistent ventilation. The study further validated that a brief educational intervention can maximize its effectiveness, making Sotair a very useful tool for EMS providers in improving patient outcomes.
Reference:
Varone FA, Brady MF. Development and validation of an educational intervention to improve performance with a new manual ventilation device (Sotair™). Presented at: Military Health System Research Symposium (MHSRS); 2023; Kissimmee, FL. Available at: https://mtec-sc.org/wp-content/uploads/2023/09/SafeBVM-MHSRS-2023.pdf. Accessed March 7, 2025.
1. Prolonged Manual Ventilation Simulation
ClinicalTrials.gov ID NCT06805838
View Study
Brief Summary:
A pilot study on simulated lung scenarios using the standard manual resuscitator bag, flow limiting resuscitator bag, and an FDA approved flow rate limiting device paired with a standard manual resuscitator.
2. Superiority Trial Between Sotair® Device Attached to Manual Resuscitator Versus Ventilation Alone
ClinicalTrials.gov ID NCT06261619
View Study
Brief Summary:
Effective respiratory ventilation is achieved by moving the right amount of air to and out of the lungs while keeping the pressures at a safe level. A disposable safety device, Adult Sotair®, was created to improve manual ventilation delivery. In this superiority study, the investigators will perform two-group cross over randomized design to test the superiority of the Adult Sotair® device compared to manual ventilation alone.
3. Non-inferiority Trial Between Sotair® Device Attached to Manual Resuscitator Versus Mechanical Ventilation
ClinicalTrials.gov ID NCT06117683
View Study
Brief Summary:
Effective respiratory ventilation is achieved by moving the right amount of air in and out of the lungs while keeping the pressures at a safe level. A disposable safety device, Adult Sotair®, was created to improve manual ventilation delivery. In this non-inferiority study, we will perform a pre-post study design (single group, within-group comparison) to test the non-inferiority of the Adult Sotair® device compared to mechanical ventilation.