SotairIQTM — Advanced Manual Ventilation Training
Sotair® — Flow-Guided Ventilation for Clinical Use
4X Increase in OHCA survival w/ adequate ventilation (1,976 pts) 7% Decrease OHCA survival w/ small adult BVMs (1,994 pts)
2.44X Increase in survival w/ compression + ventilation CPR vs. compressions only
The 3 H-Bombs: Hypoxia, Hypotension, Hyperventilation increase mortality significantly:
Severe TBI patients with field airway interventions saw 6.44X higher survival to admission and 3.52X to discharge with guidelines.


Manual ventilation is the standard method of providing emergency ventilatory support, yet it remains highly variable—even among well-trained and experienced providers.
High-stress emergencies and limited safeguards make manual ventilation prone to errors: too fast (hyperventilation), too much volume (over-ventilation), too slow (hypoventilation), or too little volume (underventilation), all of which increase the risk of serious complications such as aspiration pneumonia, lung injury, Acute Respiratory Distress Syndrome (ARDS), and even death.
Of responders delivered at least one breath that was inadequate or excessive per 90-second period.8
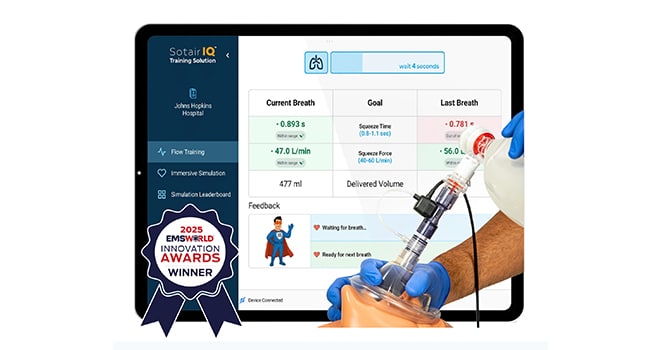
Master Flow,
Master Manual Ventilation

Flow Control Safety for Manual Ventilation












