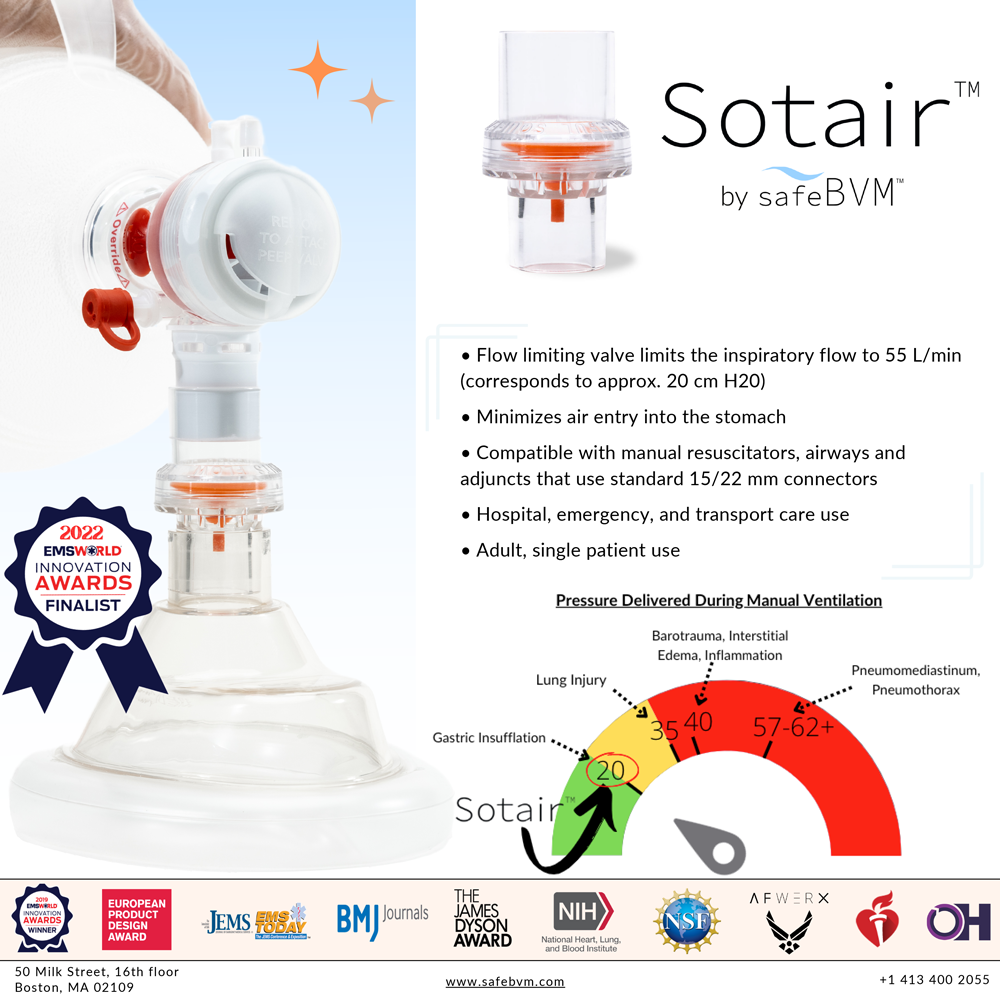
SafeBVM’s first product, the ADULT Sotair™ Device, was recently FDA 510(k) cleared and will be available for purchase at the end of 2022. Sotair limits the high flow rates delivered from the manual resuscitator, minimizing complications such as gastric insufflation that arise from over-ventilation.