SafeBVM’s flagship innovation, the award-winning Sotair® device, is primarily funded by grants and contracts from the National Institutes of Health (NIH), the National Science Foundation (NSF), and the U.S. Military.
Sotair is a small, lightweight, easy-to-use flow control valve that fits between a manual resuscitator and a patient mask or airway. The valve prevents inspiratory flow rates from exceeding 55 LPM during manual ventilation, helping keep air delivery within safer limits without added cognitive load—no dials, screens, or additional equipment—allowing providers to stay focused on the patient.
By regulating flow, Sotair helps reduce peak airway pressures, excessive tidal volumes, and excessive breath delivery rates, minimizing the risk of over-pressurization, over-ventilation, and hyperventilation.
Sotair’s user-friendly design requires approximately 30 seconds of training and provides multi-sensory feedback (haptic, auditory, and visual) to guide providers in real time—improving consistency and reducing variability during manual ventilation.

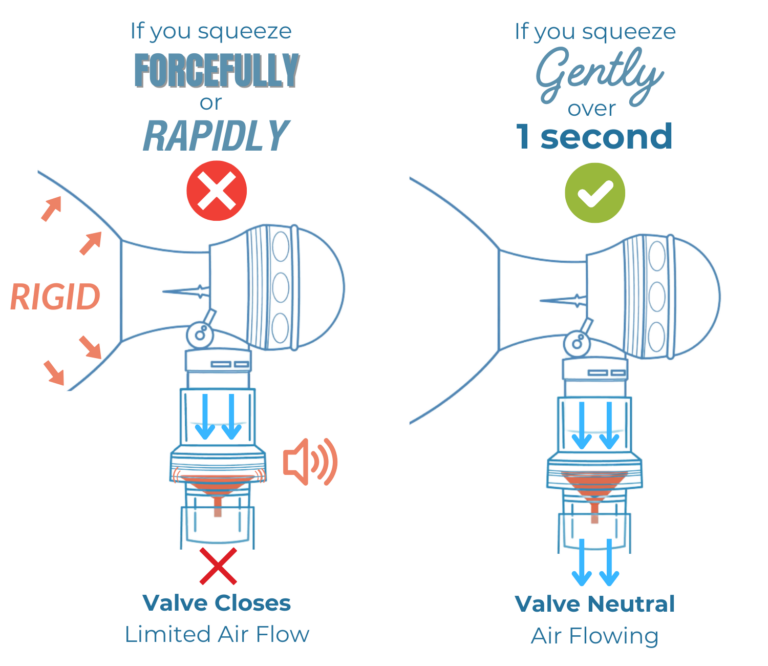
If a manual resuscitator is squeezed too fast or too forcefully and flow exceeds 55 LPM, Sotair’s valve closes, creating resistance and triggering tactile and auditory feedback alarms with visual cues. This feedback helps the provider correct their squeeze and deliver ventilation tailored to the patient’s lung condition.
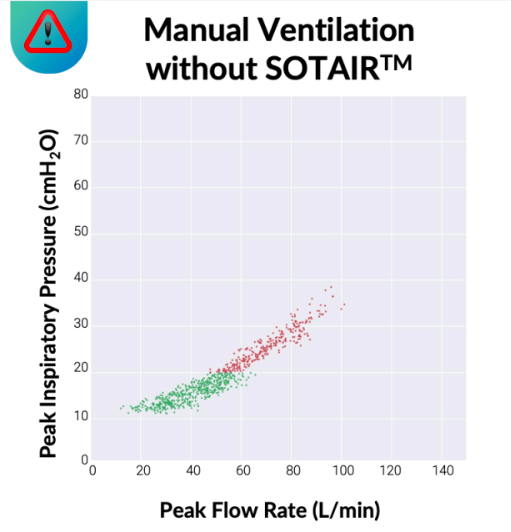
Sotair has a fixed inspiratory flow limit of approximately 55 LPM, but the pressure delivered to the patient is patient-dependent, varying with lung compliance and airway resistance.
In an average adult with healthy lungs, this flow limit typically corresponds to peak airway pressures around ~20 cmH₂O. In disease states such as COPD, asthma, or ARDS—where lung compliance is reduced and/or airway resistance is increased—higher peak pressures (often in the 20–30+ cmH₂O range) may be required to deliver effective ventilation.
Unlike traditional pressure-relief (pop-off) valves, which vent gas when higher pressure is needed—such as during airway obstruction or chest compressions—Sotair® preserves delivered volume while keeping pressures within safer limits.
When used with supraglottic airways, this controlled pressure delivery helps maintain the airway seal, improving ventilation safety while bringing SGAs closer to a functional definitive airway.
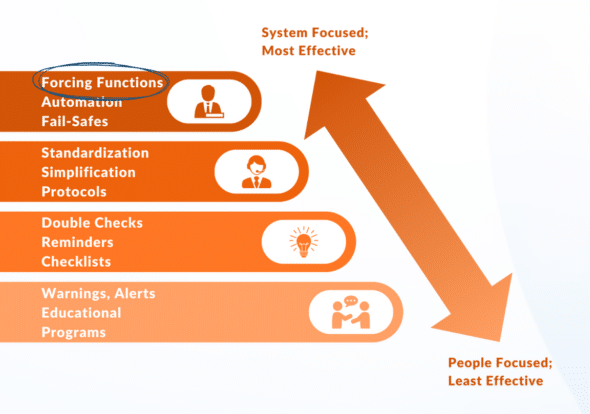
By acting as a ‘forcing function,’ Sotair® forces the right actions, avoiding rapid, forceful squeezes that lead to over-ventilation.
During the COVID-19 pandemic, a ventilator shortage was anticipated, making manual ventilation a potential alternative. 47 volunteers completed two one-hour manual ventilation sessions, randomized to start with or without Sotair.
Sotair reduces variability in manual ventilation, bringing performance closer to mechanical ventilators.
Authors concluded that extended manual ventilation may be feasible with a safety device, which may reduce barotrauma, under-ventilation and over-ventilation.7
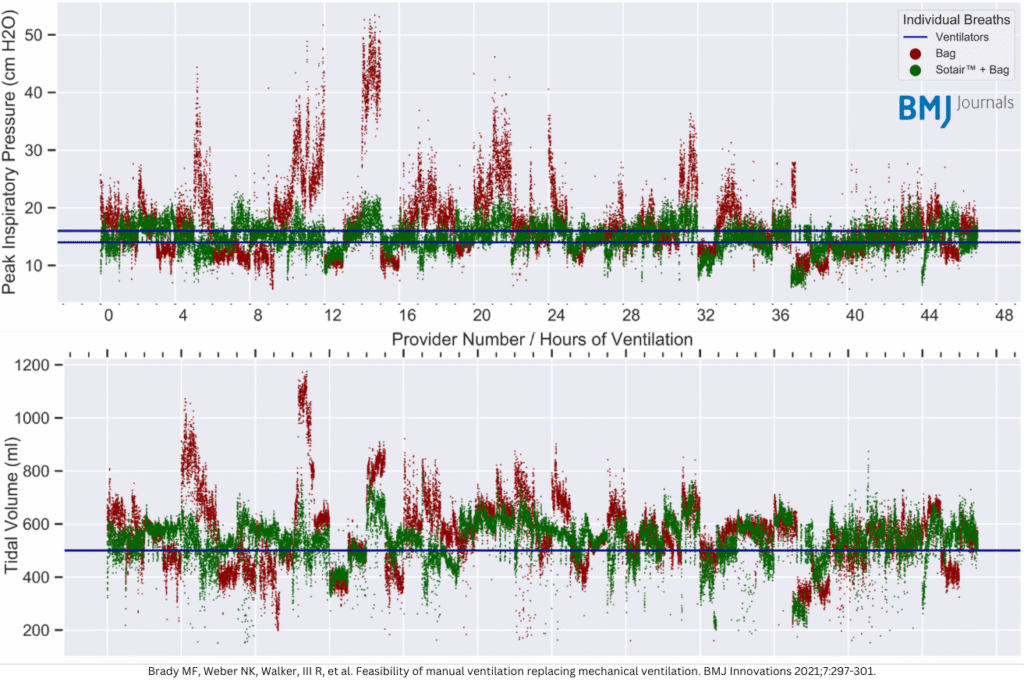
Peak pressures (p>.0001) and tidal volumes (p>0.0001) were significantly improved with the Sotair® device.
Grant 1U54HL143541 (NHLBI) funded a 40-provider pilot bench evaluation of Sotair® with North Providence Fire Station. Implementation of Sotair® showed a 36% improvement in manual ventilation.11
