

Introduction to Sotair®
SafeBVM’s flagship innovation, the award-winning Sotair® device, is primarily funded by grants and contracts from the National Institutes of Health (NIH), National Science Foundation (NSF), and the Military.
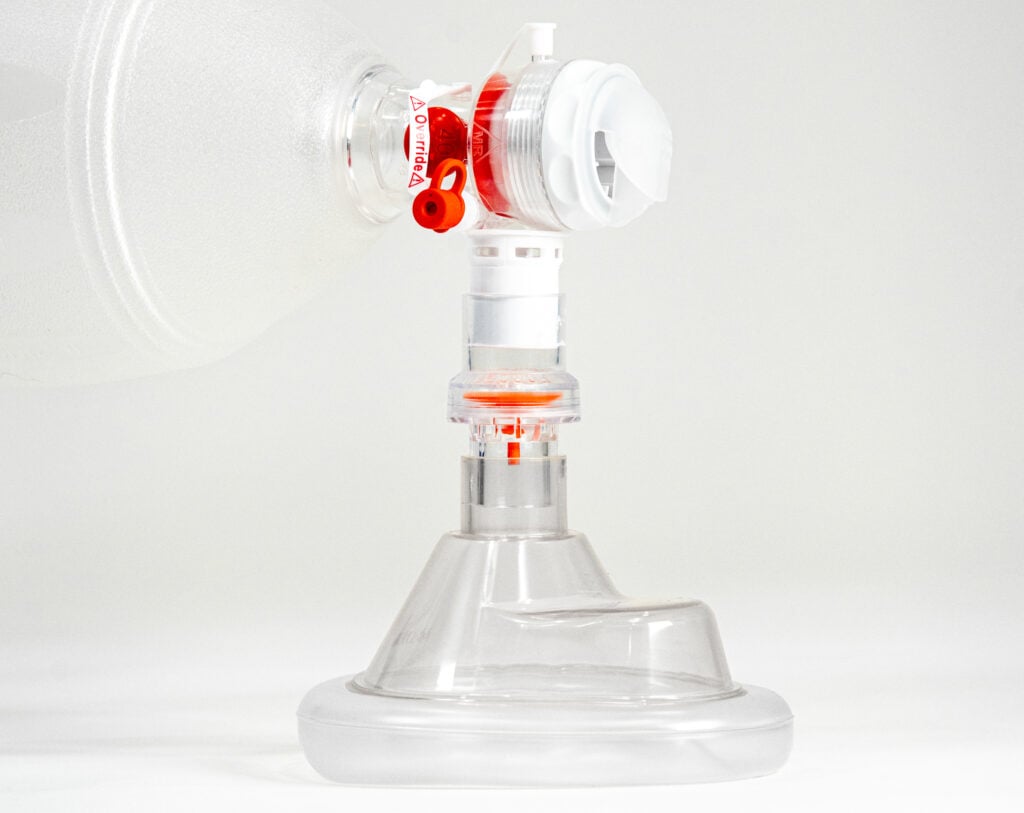
Sotair is a small, lightweight, easy to use flow control valve that fits between a manual resuscitator and a patient mask or airway. The valve prevents flow rates of air from exceeding 55 LPM during manual ventilation, ensuring that air delivery stays within safe limits. Flow control helps reduce peak airway pressures, excessive tidal volumes and excessive breath delivery rates, minimizing the risk of over-pressurization, over-ventilation and hyperventilation.
Sotair’s user-friendly design requires only 30 seconds of training. The device offers multi-sensory feedback (haptic, auditory, visual) to guide providers in real-time, enhancing adaptability and consistency while reducing variability in performance during manual ventilation.
Features & Benefits
Fits between a manual resuscitator and a patient mask/airway
Flow rates of air are limited to 55 LPM
Regulation of airflow helps reduce peak airway pressures, excessive tidal volumes¹ and excessive breath delivery rates,² minimizing the risk of over-pressurization, over-ventilation and hyperventilation
Peak Pressure limit during air delivery is automatically adjusted based on the patient’s lung compliance and resistance
Acts as a ‘Forcing Function,’ constraining the provider to deliver safer manual ventilation with less variability. This is most effective in ensuring system-wide change
Immediate real-time tactile and auditory alarms with visual cues
Decreases provider variability and improves consistency of ventilation
Reliable performance: 100% activation and flow limiting testing
30 second basic training
FDA 510(k) cleared for adult, single patient use
How Does Sotair® Work?
If the bag is squeezed too quickly or forcefully causing inspiratory flow to exceed 55 LPM, Sotair’s valve closes, creating a resistance in the bag producing alarms realized by the user. Alarms are tactile and/or auditory with visual cues and prompts the user to adjust their technique encouraging the right actions, avoiding rapid, forceful squeezes that lead to unsafe ventilation. This intuitive feedback makes training easy and reinforces best practices tailored to different patient lung conditions during real-time clinical use.
Dynamic Pressure Delivery
While Sotair has a static flow limit of ~55 LPM, the output peak pressure delivered to the patient is dynamic depending on the patients lung compliance and resistance. For example, for an average adult with healthy lungs, Sotair’s flow limit of 55 LPM corresponds to a maximum peak pressure delivered of approximately 20 cmH2O, when air starts to enter the stomach. In other the disease states like COPD, Asthma or ARDS, the peak pressure generated by the user may be in the 20-30+ cmH2O as they have decreased lungs compliance and/or increased airway resistance.



British Medical Journal, Innovations
During the COVID-19 pandemic, a ventilator shortage was anticipated, making manual ventilation a potential alternative. 47 volunteers completed two one-hour manual ventilation sessions, randomized to start with or without Sotair.
Sotair reduces variability in manual ventilation, bringing performance closer to mechanical ventilators.
Authors concluded that extended manual ventilation may be feasible with a safety device, which may reduce barotrauma, under-ventilation and over-ventilation.10

Peak pressures (p>.0001) and tidal volumes (p>0.0001) were significantly improved with the Sotair® device.
GRANT-FUNDED CASE STUDY
Pre-Post Sotair® Implementation at a Fire & EMS Service



Testimonials
MD FACEP FAEMSMedical Director of the State of Tennessee EMS,
Associate Professor in Emergency Medicine at University of Tennessee Health Science Center

EdD, RRT-NPS, FAARCChairman, Department of Respiratory Therapy,
Georgia State University
