SafeBVM & Sotair/SotairIQ: Aligne...

Ventilation Fundamentals 2025 AHA Guidelines: Recommend delivering each breath over one second. Audiovisual feedback devices have been shown to improve ROSC and survival to discharge from cardiac arrest. Delivering a breath over 1 second underscores that effective ventilation depends not only on volume but also on how that volume is delivered, through controlled flow and proper timing […]
The ADULT Sotair Device Receives 510 (...
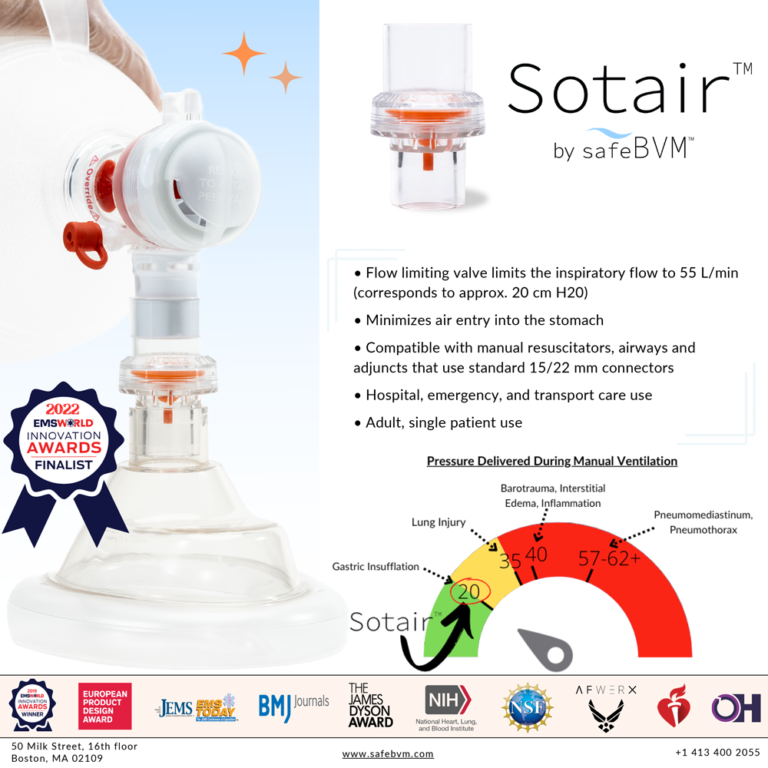
SafeBVM’s first product, the ADULT Sotair™ Device, was recently FDA 510(k) cleared and will be available for purchase at the end of 2022. Sotair limits the high flow rates delivered from the manual resuscitator, minimizing complications such as gastric insufflation that arise from over-ventilation. Posted in Sotair News

